BridgeBio Pharma Reports Third Quarter 2019 Financial Results and Highlights Portfolio Progress
-Multiple clinical and pre-clinical milestones achieved
across the BridgeBio portfolio
-Delivered pipeline growth with the addition of BBP-418 for
limb-girdle muscular dystrophy type 2i
-Ended quarter with $446.1 million in cash, cash equivalents
and marketable securities, excluding Eidos
Recent Highlights:
- BBP-831 – FGFR1-3 inhibitor for achondroplasia: Initiated PROPEL, a prospective observational study in children with achondroplasia, the most common genetic form of short stature (NCT04035811). The study will establish annualized growth velocity (AGV) for each child over a minimum period of six months. PROPEL is designed to provide baseline measurements for children who enroll in PROPEL2, a Phase 2 study of low-dose infigratinib in achondroplasia which is on track to start in 2020. Additionally, in October 2019, new preclinical data supporting tolerability and efficacy of infigratinib in the mouse model of achondroplasia were reported at the American Society of Human Genetics conference (link to poster).
- BBP-870 – cPMP replacement therapy for MoCD type A: Presented natural history study data at the Society of Inborn Errors of Metabolism Conference (link to poster) in September. These data suggest an urgent need for new therapies in molybdenum cofactor deficiency (MoCD) type A, an often-fatal rare genetic disease, and will be a critical component of the planned new drug application.
- BBP-812 – Gene therapy candidate for Canavan disease: Opened a natural history study in Canavan disease (treatcanavan.com). Presented preclinical data (link to poster) demonstrating intravenous (IV) dosing of BridgeBio’s experimental therapy for Canavan disease (BBP-812) achieved broad central nervous system delivery; the IV approach is much less invasive compared to intrathecal or intracerebroventricular alternatives.
- BBP-631 Gene therapy candidate for CAH: Presented preclinical update (link to poster) for gene therapy candidate BBP-631 in congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency wherein IV dosing of non-human primates with BBP-631 resulted in durable delivery and expression of the gene product to the adrenal tissue.
- BBP-398 – SHP2 inhibitor for treatment-resistant cancer: Presented data (link to poster) highlighting the discovery and preclinical efficacy of BBP-398, a potent and selective SHP2 inhibitor, which is currently being prepared for the submission of an IND in 2020 for evaluation in RTK-driven cancer.
- BBP-265 (AG10) – TTR stabilizer for ATTR: Granted Alexion Pharmaceuticals, Inc. an exclusive license to develop and commercialize AG10 in Japan for an upfront payment of $25 million and an equity investment of $25 million.
- Pipeline growth: Announced addition of a new asset, BBP-418, to the pipeline. BBP-418 is a substrate supplementation therapy for the treatment of limb-girdle muscular dystrophy type 2i and is in IND-enabling studies. This condition affects an estimated 7,000 patients in the United States and European Union with no currently approved therapies.
- Organizational growth: Added two new members to the senior leadership team. Brian Stolz joined as Chief Operating Officer of BridgeBio. Mr. Stolz most recently held the position of Chief People Officer at Activision Blizzard. Yi Ching Yau joined as Chief Accounting Officer of BridgeBio. Prior to joining BridgeBio, Ms. Yau served as the VP of Finance at Nektar Therapeutics.
Upcoming Milestones:
- BBP-265 (AG10) – TTR stabilizer for ATTR: Plan to present interim analysis of the ongoing Phase 2 open label extension study (NCT03536767) of AG10 in patients with TTR amyloid cardiomyopathy, an inherited form of heart failure, at the American Heart Association 2019 Scientific Sessions in a Late-Breaking Featured Science Oral Presentation. A Phase 3 study of AG10 in ATTR-PN (ATTRibute-PN) is on track to begin in the first quarter of 2020.
- BBP-870 – cPMP replacement therapy for MoCD type A: On track to initiate a rolling new drug application submission for our first-in-class therapy for molybdenum cofactor deficiency (MoCD) type A, BBP-870, by the end of 2019.
- BBP-589 – COL7A protein replacement therapy for recessive dystrophic epidermolysis bullosa: Plan to share topline data from the ongoing Phase 1/2 study (NCT03752905) during 2020.
- BBP-831 (infigratinib) – FGFR1-3 inhibitor for FGFR2+ cholangiocarcinoma: Plan to complete enrollment of the ongoing pivotal Phase 2 study (NCT02150967) in second line cholangiocarcinoma (bile duct cancer) and present updated results at a major oncology meeting in 2020. Remain on track to submit new drug application for FDA approval in 2020.
Third Quarter 2019 Financial Results:
Cash, Cash Equivalents and Marketable securities
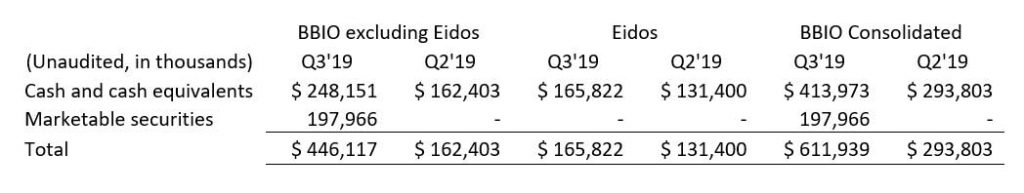
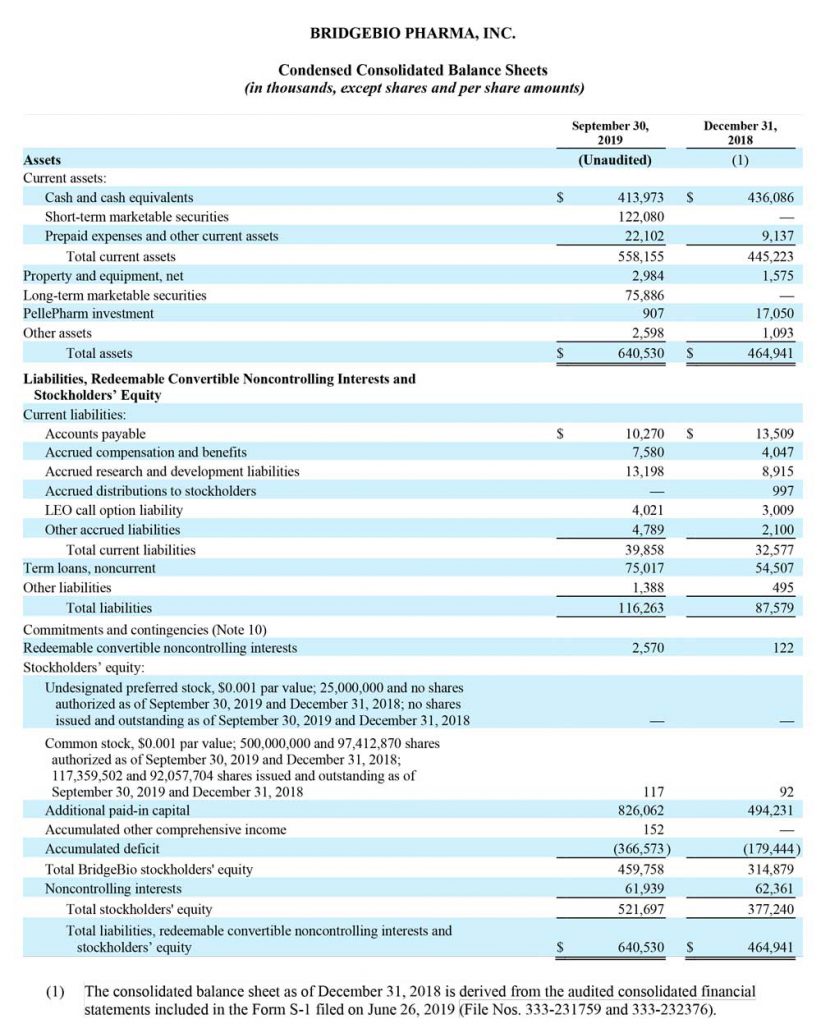
Consolidated cash, cash equivalents and marketable securities, excluding restricted cash, totaled $611.9 million as of September 30, 2019. Excluding Eidos, BridgeBio’s cash balance as of September 30, 2019 was $446.1 million compared to $162.4 million as of June 30, 2019. The net change in cash balance of $283.7 million reflects net proceeds received from BridgeBio’s initial public offering of $368.7 million, offset by the repurchase of a non-controlling interest for $26.4 million and approximately $58.6 million primarily for operating expenses.
Operating Expenses
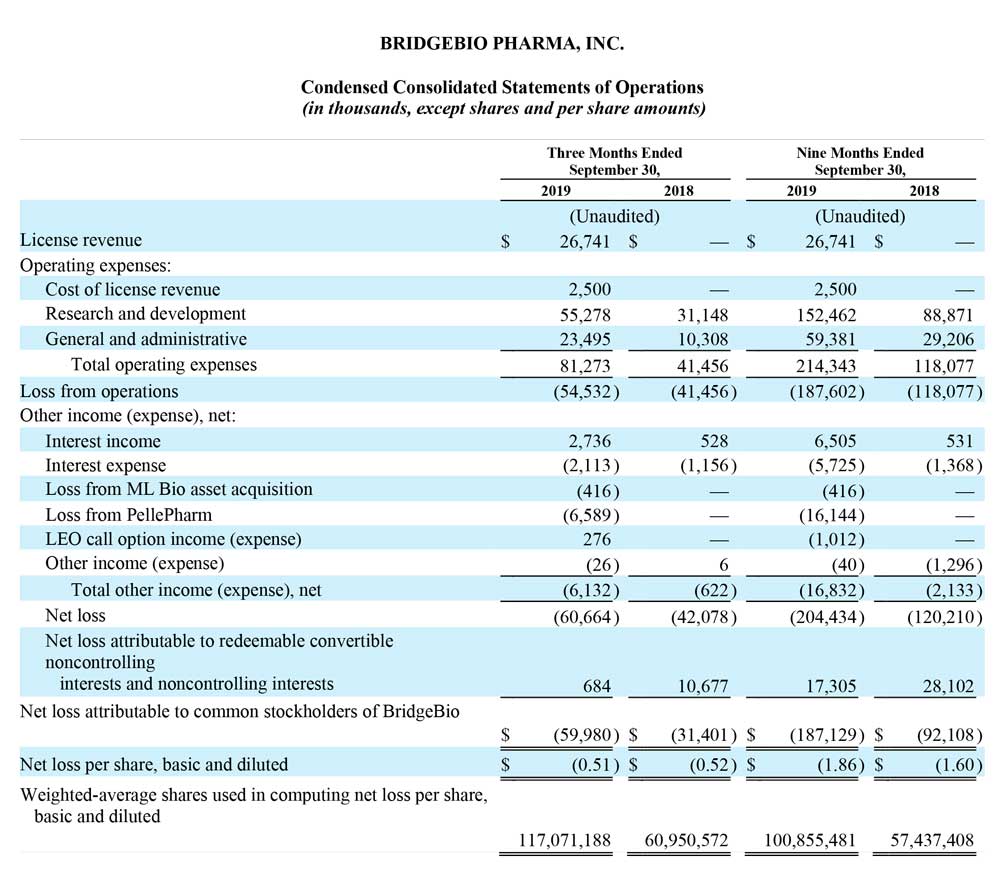
Operating expenses for the three months that ended September 30, 2019 were $81.3 million, as compared to $41.5 million for the same period in the prior year. The increase in operating expenses of approximately $39.8 million was mainly attributable to increased research and development expenses related to the progression of our programs.
About BridgeBio Pharma
BridgeBio is a team
of experienced drug discoverers, developers and innovators
working to create life-altering medicines that target
well-characterized genetic diseases at their source. BridgeBio
was founded in 2015 to identify and advance transformative
medicines to treat patients who suffer from Mendelian diseases,
which are diseases that arise from defects in a single gene, and
cancers with clear genetic drivers. BridgeBio’s pipeline
of over 15 development programs includes product candidates
ranging from early discovery to late-stage development. For more
information, please visit www.bridgebiodev.wpengine.com.
BridgeBio Pharma Forward-Looking Statements
This press release contains forward-looking statements.
Statements we make in this press release may include statements
which are not historical facts and are considered
forward-looking within the meaning of Section 27A of the
Securities Act of 1933, as amended, and Section 21E of the
Securities Exchange Act of 1934, as amended, which are usually
identified by the use of words such as
“anticipates,” “believes,”
“estimates,” “expects,”
“intends,” “may,” “plans,”
“projects,” “seeks,”
“should,” “will,” and variations of such
words or similar expressions. We intend these forward-looking
statements to be covered by the safe harbor provisions for
forward-looking statements contained in Section 27A of the
Securities Act and Section 21E of the Securities Exchange Act
and are making this statement for purposes of complying with
those safe harbor provisions. These forward-looking statements,
including statements relating to the clinical and therapeutic
benefits of our product candidates, our ability to initiate
additional preclinical studies and clinical trials, our ability
to submit planned regulatory filings for our product candidates,
our ability to generate data from our ongoing and planned
preclinical studies and clinical trials, and the timing of these
events, reflect our current views about our plans, intentions,
expectations, strategies and prospects, which are based on the
information currently available to us and on assumptions we have
made. Although we believe that our plans, intentions,
expectations, strategies and prospects as reflected in or
suggested by those forward-looking statements are reasonable, we
can give no assurance that the plans, intentions, expectations
or strategies will be attained or achieved. Furthermore, actual
results may differ materially from those described in the
forward-looking statements and will be affected by a variety of
risks and factors that are beyond our control including, without
limitation, our ability to continue our planned research and
development activities and complete our planned regulatory
submissions, as well as those set forth in the Risk Factors
section of BridgeBio Pharma Inc.’s most recent Quarterly
Report on Form 10-Q and our other SEC filings. Except as
required by law, we assume no obligation to update publicly any
forward-looking statements, whether as a result of new
information, future events or otherwise.
Contact:
Alberto Gestri
[email protected]